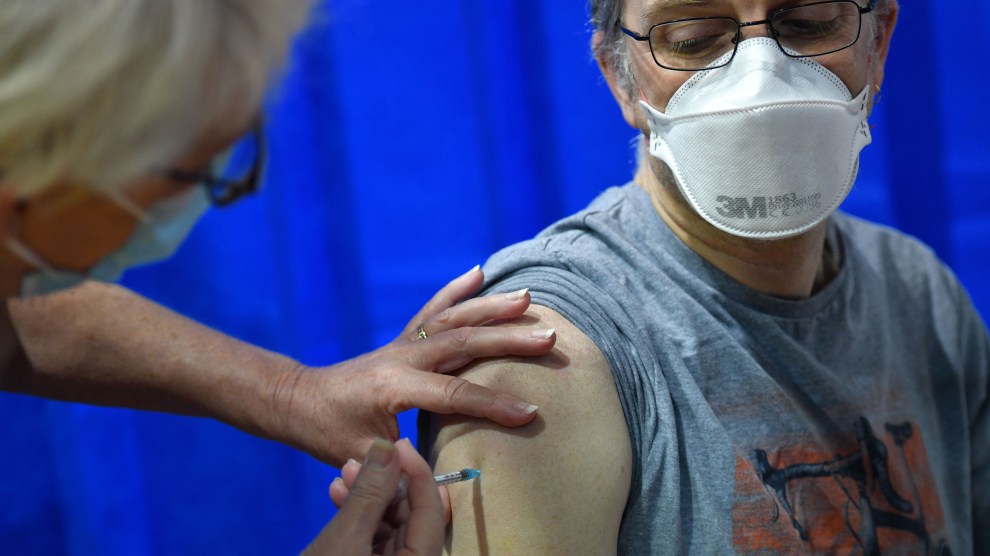
A nurse administers the Pfizer vaccine at a health center in Wales.Pool/i-Images/Zuma
Update: Dec. 11, 9:30 p.m. ET: The FDA granted emergency use authorization for Pfizer’s coronavirus vaccine Friday night, in a potentially historic turning point for the fight against the global pandemic. Earlier Friday, White House Chief of Staff Mark Meadows told FDA commissioner Stephen Hahn that he should resign if the vaccine was not approved by the end of the day. The approval will immediately pave a way for immunizing at least some of the most needy demographics in a country currently beset by rocketing COVID infection rates, with daily fatality rates rivaling that of 9/11.
In a major milestone that brings the United States one step closer to the end of the coronavirus pandemic, a Food and Drug Administration advisory panel recommended on Thursday that the FDA grant an emergency use authorization for Pfizer’s COVID vaccine.
The group of scientists and doctors voted 17–4 in favor of the vaccine’s approval, with one abstaining. The FDA is expected to approve the vaccine within days, the New York Times reports, and within 24 hours after approval 6.4 million doses will begin to be distributed from warehouses.
Pfizer’s vaccine is estimated to be about 95 percent effective, and it has already been approved in the United Kingdom and Canada. It’s not yet clear how many doses of Pfizer’s will be immediately available or when most Americans can expect to be vaccinated. The Moderna vaccine, another option, is under review by the FDA.