Getty
Inflammation, itching, painful skin lesions: This is everyday life for patients with atopic dermatitis, a type of eczema that affects roughly 11 percent of adults and 7 percent of children in the United States and accounts for nearly $5.3 billion in annual health care costs. Topical steroids, usually hydrocortisone ointments, are often the first line of defense against eczema. The list price for a jar of such ointment can exceed $100—and it usually needs to be applied daily.
“Living with atopic dermatitis can be physically and emotionally challenging,” Anthony S. Fauci, director of the National Institute of Allergy and Infectious Diseases, said in a statement. “Currently available therapies can be time-consuming—requiring multiple daily applications—and costly.”
Indeed, skincare companies target eczema patients with expensive lotions, oils, and other products. Even within the health care industry, a 2017 study found, outpatient care and prescription costs add up to thousands of dollars each year for patients with atopic dermatitis.
Now, scientists have identified a potentially cheap and effective alternative: bacteria.
In an early-phase clinical trial at the National Institutes of Health, the bacterium Roseomonas mucosa reduced the severity of atopic dermatitis in adults and children. The bug, naturally present on human skin, was previously shown to improve symptoms in mice. The ongoing trial is the first time it has shown results in human patients.
The study, published Thursday in JCI Insight, shows early results for 10 adults and 5 children with atopic dermatitis. Researchers harvested R. mucosa bacteria from healthy volunteers and grew it under lab conditions. Then, twice a week for six weeks, the adults sprayed a solution of sugar water and R. mucosa onto their inner elbows, one of the problem spots for the disease. None of the adult volunteers reported any complications, most reported improvements, and later, some reported less need for topical steroids. Similar results were seen with the children, who received applications of the bacterial solution twice a week for 12 weeks, and then every other day for 4 weeks.
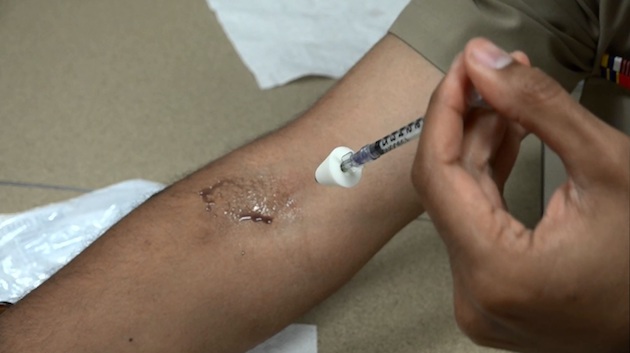
A scientist demonstrates application of the experimental therapy to the inner elbow. Here the bacterial solution has been replaced with a purple dye.
NIAID
The NIH researchers chose R. mucosa in a bid to alter the skin’s microbiome, the delicate balance of tiny organisms that live on the surface. The results seem to suggest that microbiome-targeting interventions show real therapeutic potential, and the researchers are actively recruiting new volunteers to expand their trial.
This isn’t the first time bacteria have shown promise against skin diseases. In a study published last February, certain Staphylococcus strains inhibited the growth of Staphylococcus aureus on human skin—although a common bacterium, S. aureus is especially abundant in atopic dermatitis patients, worsening the disease’s effects and weakening the immune system.
Ian Myles, the eczema trial’s principal investigator, hopes the new bacterial treatment can eventually be a cheap alternative solution: “We hope our work will lead to development of new, low-cost atopic dermatitis therapies that do not require daily application.”