The FDA has released its briefing document for Moderna’s COVID-19 vaccine, and it looks pretty great:
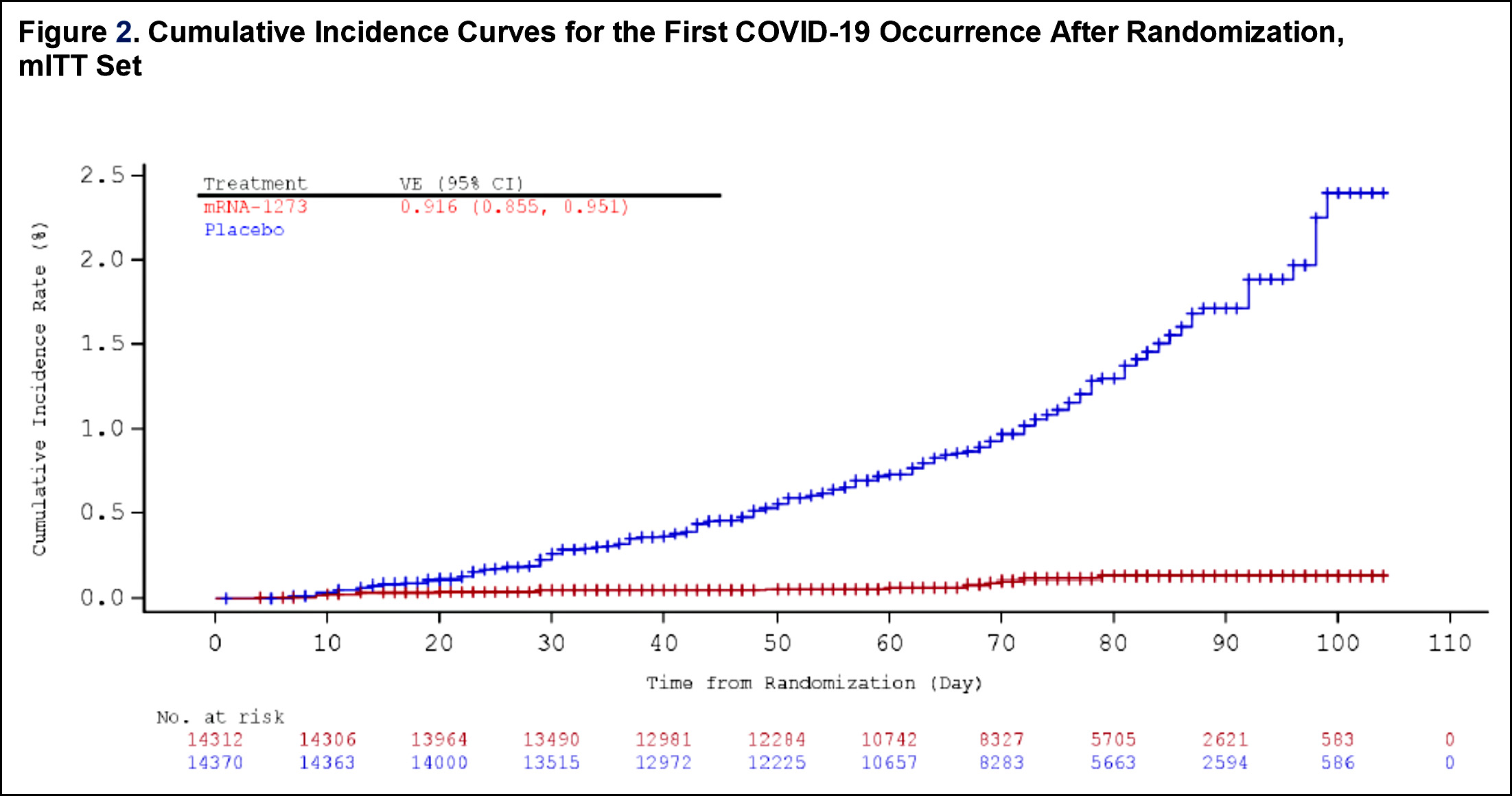
This looks even better than the Pfizer vaccine, and it has the added advantage of requiring only normal refrigeration for storage, rather than the super-cold refrigeration required by Pfizer’s product. As with the Pfizer vaccine, patients reported a fairly normal incidence of minor side-effects, including headache, fever, fatigue, muscle aches, joint pain, chills, and pain at the injection site. There were apparently no serious side effects. The Moderna product is a two-shot vaccine, with the second shot given 28 days after the first—although the chart suggests that it’s pretty effective even after the first dose. Like the Pfizer vaccine, it appears to become effective about ten days after the first injection.
One other thing of note. This might be simply a coincidence, but every single person in the vaccine group who contracted COVID-19 (five in all, compared to 90 in the control group) were white people under the age of 65. No person of color contracted COVID-19, and neither did anyone over the age of 65.
The FDA’s vaccine advisory committee meets on Thursday to discuss the results of the Moderna testing. They seem very likely to recommend an emergency use authorization, and the FDA will probably issue one on Friday. By next week we should have two highly effective vaccines in active use.